Caiaimage/Martin Barraud
The US could see the largest number of approvals for medtech devices in around 10 years given the high number of approvals the US FDA granted in the first half of 2023.
The agency’s Center for Devices and Radiological Health in H1 approved or cleared 45 new devices or products, just one fewer than in all of 2022.
However, the FDA has been taking longer to grant approvals, according to a new report from Evaluate Vantage. It found that CDRH spent an average of ~3 years to grant 22 approvals for high-risk devices, much longer compared to previous years. The pharma, biotech and medtech analysis firm noted that two of those devices had been waiting more than 10 years for approval.
Evaluate Vantage found that between 2013 and 2022, the FDA spent an average of 16.4 months to grant premarket approval applications (PMAs) and medical device exemption (MDEs) applications. For applications approved in H1 2023, the figure is double that.
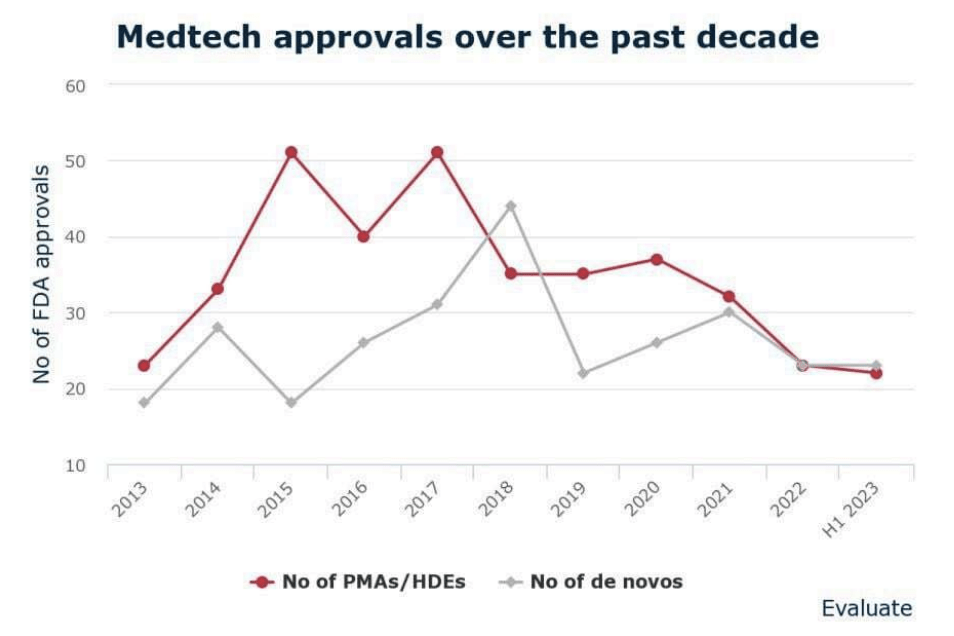
Much of the reason for the poor showing in medtech approvals last year has to with the COVID-19 pandemic, which saw the FDA examine many device applications for Emergency Use Authorization rather than traditional pathways, Evaluate Vantage said. Many of the EUAs were for diagnostic devices and ventilators.
In H1 2023, four COVID diagnostic tests have received de novo clearance. Three are from QuidelOrtho (QDEL) and one from Cue Health (HLTH).
In the first half of the year, the FDA granted approval to 22 PMAs and HDEs, and 23 de Novo applications. In vitro diagnostics saw the largest number of combined approvals (10) followed by cardiology (6).
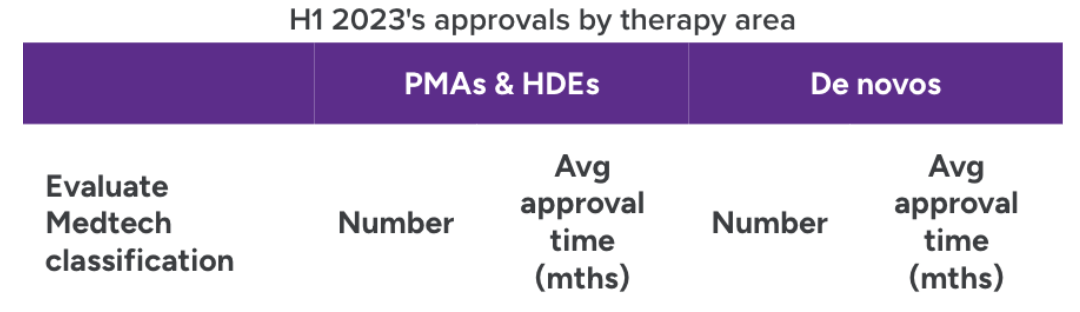
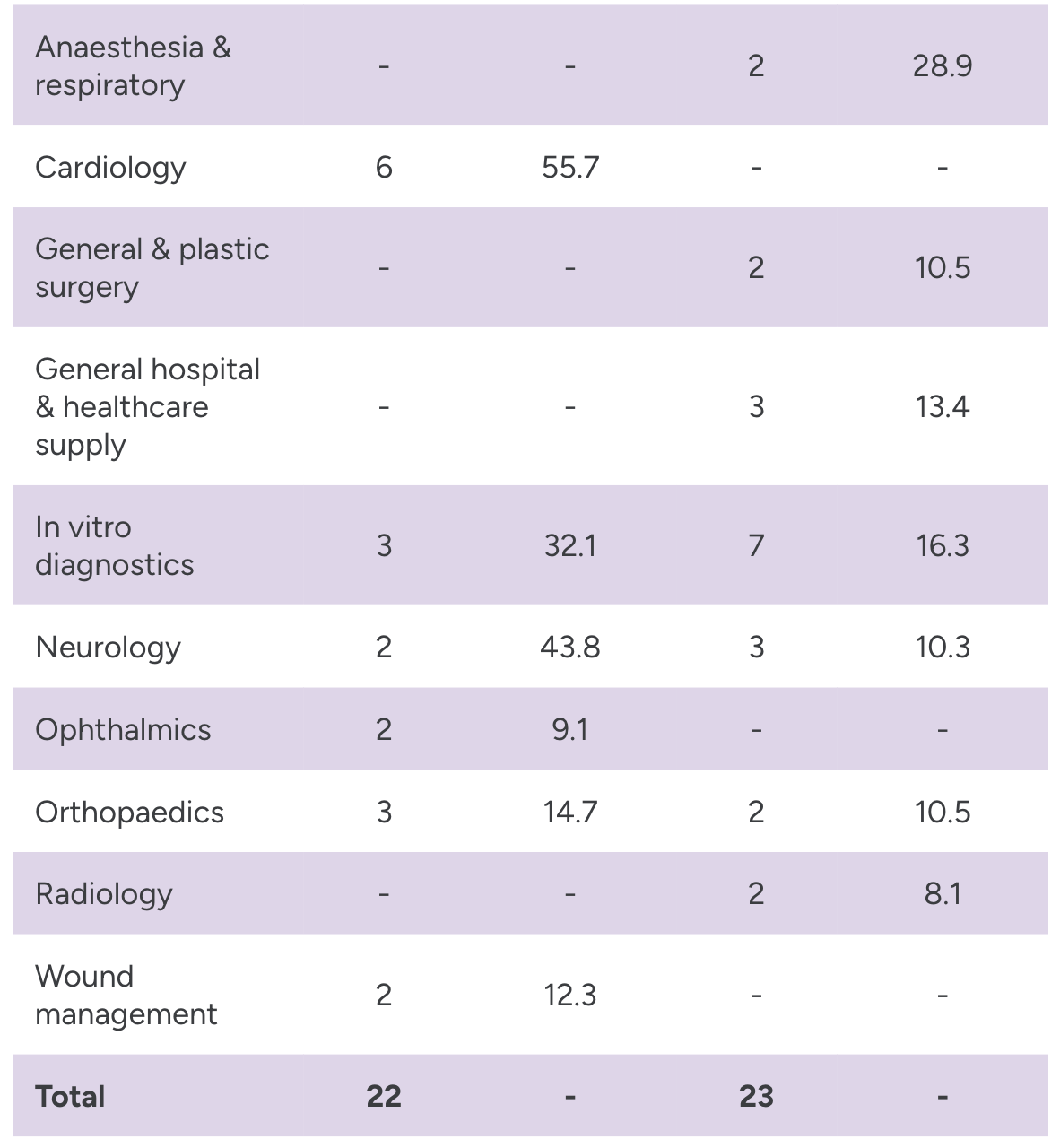
Source: Evaluate Medtech and FDA. Note: Areas with fewer than two approvals were excluded from chart.
As Evaluate Vantage pointed out, in October 2022, CDRH launched its Total Product Lifecycle Advisory Program (“TAP”), a pilot program designed to speed up approvals by improving communications between the agency and companies.
As of July 31, five devices have enrolled in TAP. In addition, beginning Oct. 1, the program will expand to include the Office of Neurological and Physical Medicine Devices.
For fiscal 2023, the program intends to enroll up to 15 devices — limited to cardiovascular — in the program. In FY 2024, that number will expand to up to 45 and not be restricted.
“The TAP will not have had time to make its effects felt yet, but it could help the agency review innovative devices more rapidly in the future,” wrote Evaluate Vantage’s Elizabeth Cairns.
Selected medtech companies: Medtronic (NYSE:MDT), Boston Scientific (NYSE:BSX), Edwards Lifesciences (NYSE:EW), and Abbott Laboratories (NYSE:ABT).
More on medtech
Medtronic: Expanding Product Portfolio Without Affecting Dividend Growth
Boston Scientific Remains Operationally Sound But May Have Limited Upside
Edwards Lifesciences: Slowly Approaching Its Intrinsic Value
Abbott: This Dividend King Is Ready For Takeoff